About TMS Treatment
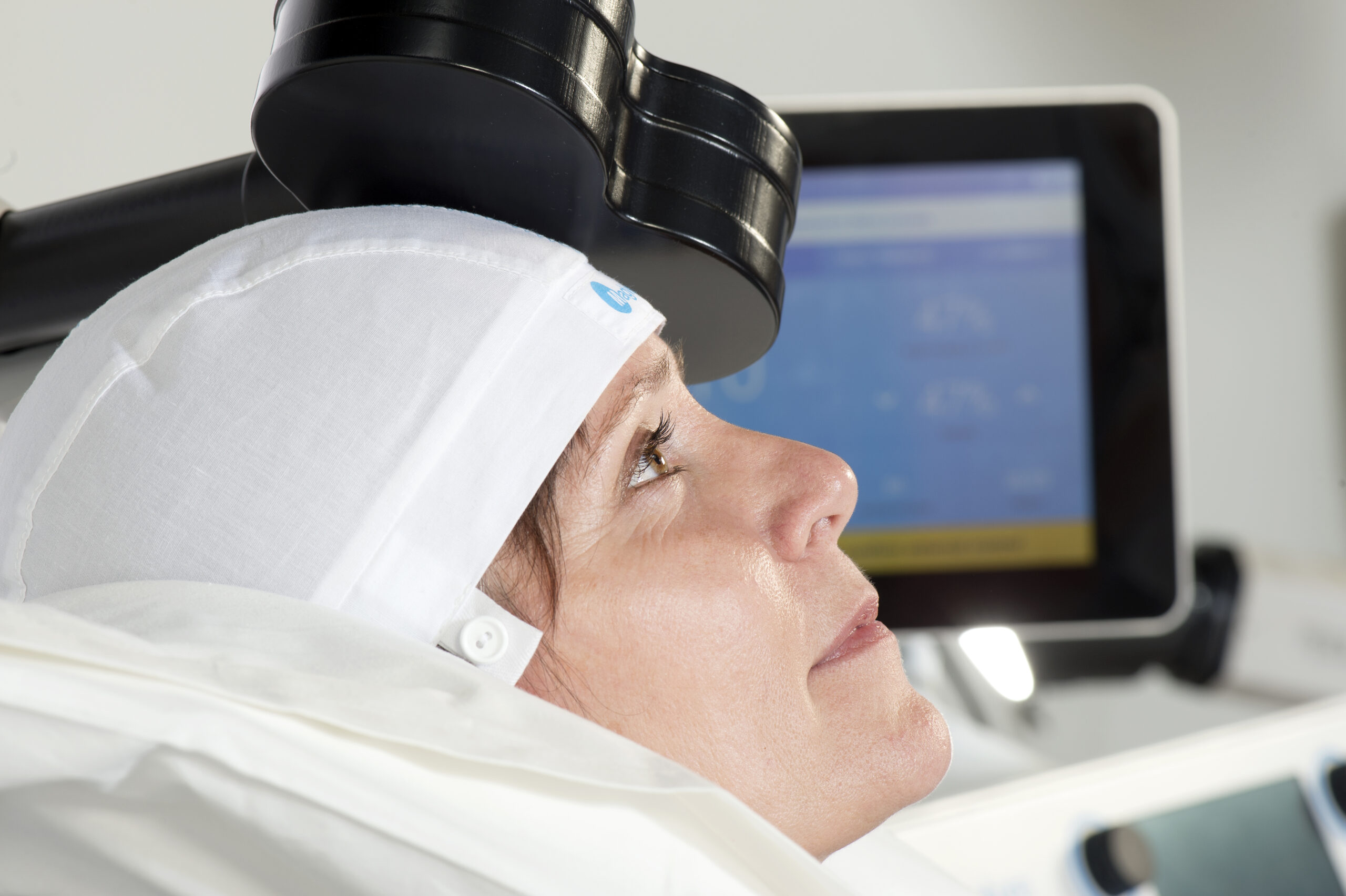
Transcranial Magnetic Stimulation (TMS) is an FDA-approved, non-invasive, non-medication, interventional therapy that uses magnetic pulses to reverse the changes in the brain that occur from depression.
In some cases depression and related conditions such as anxiety and PTSD are resistant to medication. Fortunately, due to new medical technologies, TMS treatment is effective in treating depression. Other psychiatric disorders, such as PTSD and anxiety, can benefit from TMS treatment since depression is often present in these conditions.

Unlike traditional pharmacological antidepressant therapy, TMS allows for the treatment of depression, without the associated unpleasant side effects, such as decreased libido, weight gain, and emotional blunting (inability to experience the full range of human emotions such as joy and happiness).
How does TMS work?
TMS treatment stimulates the part of the brain that is underactive in people with depression. The TMS Treatment system generates highly concentrated magnetic fields that rapidly switch on and off. These magnetic fields do not directly impact the entire brain, reaching only a few centimeters inward, directly beneath the treatment coil. By stimulating this part of the brain, the neurons in effect, “learn” to be more active, which is associated with the resumption of functioning and improvement from depression symptoms.
In some instances, TMS can be used alone to treat depression. If TMS is successful, many individuals can come off some or all of their medications, and TMS can work concurrently with therapy or on its own.
Learn more by reading our TMS FAQs.
Why Choose Us for TMS Treatment?
Qualified
Unlike some TMS providers, Dr. Penner holds a Medical Degree (MD). On top of four years of medical school at the Vanderbilt University of Medicine, Dr. Penner completed over five years of additional medical training in residency and fellowship at Harvard Medical School.
Experienced
Olympia Center for TMS & Psychiatry has administered an average of 8,000 treatments annually since 2019.
Personalized Treatment Options
Having a broad range of experience in various modalities, Dr. Penner can provide personalized recommendations encompassing multiple methods of treatment rather than a “one size fits all,” approach.
Questions?
If you have any questions please call (360) 539-1736 or message us and we’ll be glad to answer any questions and schedule an appointment.
TMS Treatment Types Offered
Olympia Center for TMS and Psychiatry provides both standard repetitive transcranial magnetic stimulation (rTMS) treatment, which lasts 19 minutes as well as the newer theta burst stimulation in which treatments are only 3 minutes and 26 seconds in length. Both forms of TMS have excellent outcomes in terms of safety and efficacy. The protocol chosen for each patient is specific to their needs and may be adjusted during treatment.
Effectiveness and Response Rates for TMS
Response varies widely by patient. Some patients notice improvements from the first session, such as increased focus and motivation. Typically, by the end of the 2nd week patients notice relief of symptoms to some degree.
Patients may experience rapid response and lessening of symptoms, or a gradual improvement over the entirety of treatment. Occasionally, treatment ends without significant improvement of symptoms only for patients to notice an improvement weeks after the final session.
The effects of treatment can take 6 full weeks after the last session to become apparent. For this reason, patients follow-up with the provider 6 weeks after their final session to discuss next steps and overall symptom improvement.
According to the National Library of Medicine, data shows a 63% remission rate (a substantial improvement in symptoms) and an 82% response rate (improvement of symptoms to some degree.).
TMS is FDA Approved
The current use of electromagnetic induction for transcranial stimulation dates back to 1985. Dr. Tony Barker and his colleagues invented the initial type of TMS in Sheffield, UK. Moreover, Barker scientifically proved the influence of magnetic stimulation on the motor cortex of the human brain in 1985.
In 2002, the Canadian Association of Health approved the medical results and benefits of TMS. Many devices are available for repetitive electromagnetic stimulation. Olympia Center for TMS & Psychiatry, uses the MagVita TMS Treatment System with Theta Burst Stimulation.
The Food and Drug Administration of the United States granted approval for TMS as a treatment for depression on October 8th, 2008.
